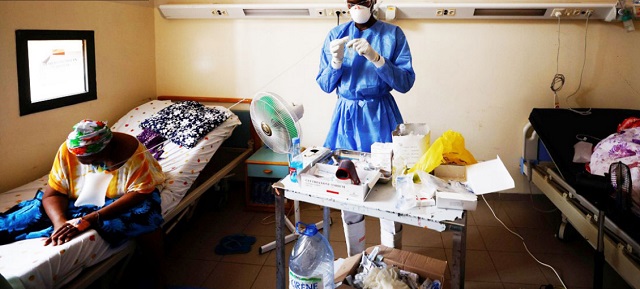
In a bid to stave off looming disaster, scientists are trying to repurpose drugs used for malaria and other diseases, but infrastructure and recruitment challenges stymie progress
| ABDULLAHI TSANNI | For more than a year, Adeola Fowotade struggled to recruit people to a clinical trial for COVID-19 treatments. A clinical virologist at University College Hospital in Ibadan, Nigeria, she joined the effort in August 2020, which aims to test the efficacy of a combination of readily available drugs. Her goal was to find 50 volunteers — people diagnosed with COVID-19 who had moderate to severe symptoms and might benefit from the drug cocktail. But the recruitment effort crawled along, even as cases of the virus surged in Nigeria in January and February. After 8 months, she had managed to enlist only 44 people.
“Some patients declined to participate in the study when approached, while some who agreed discontinued midway into the trials,” says Fowotade. Once case rates started to decline in March, it became nearly impossible to find participants. This made the trial, known as NACOVID, difficult to complete. “We were unable to meet our planned sample size,” she says. The trial ended in September, short of its recruitment goal.
Fowotade’s troubles mirror those faced by other trials in Africa — posing a major problem for those countries in the continent that have been unable to secure enough vaccines against COVID-19. Only 2.7% of people in Nigeria, the continent’s most populous country, have been at least partially vaccinated. That’s just slightly lower than the average rate for low-income countries. Estimates suggest that it could take until at least September 2022 for African nations to obtain enough doses to fully vaccinate 70% of the continent’s population.
That leaves few options for fighting the pandemic now. Although wealthy nations outside Africa have used treatments such as monoclonal antibodies or the antiviral drug remdesivir, these need to be administered in hospitals and are expensive. The drug giant Merck has agreed to license its pill-based drug molnupiravir to manufacturers that would provide broad access to the drug, but questions remain about how costly it will be if it does get approval. So the hunt is on for affordable, readily available drugs for Africa that could reduce COVID-19 symptoms, lower the burden of disease on health-care systems and reduce deaths.
COVID spurs Africa vaccines revolution
That search has faced numerous hurdles. According to the US-run database clinicaltrials.gov, of nearly 2,000 trials currently exploring drug treatments for COVID-19, only about 150 are registered in Africa, and the vast majority of those are in Egypt and South Africa.
The lack of trials is problematic, says Adeniyi Olagunju, a clinical pharmacologist at the University of Liverpool, UK, and the principal investigator for NACOVID. If Africa is largely missing from COVID-19 treatment trials, then the likelihood of it having access to drugs that get approved is very limited, he says. “Add that to the abysmally low access to vaccines,” Olagunju says. “Africa needs effective therapeutics for COVID-19 as an option more than any other continent.”
Some organisations are trying to remedy this shortfall. ANTICOV, a programme run by the non-profit Drugs for Neglected Diseases initiative (DNDi), is currently the largest trial in Africa. It is testing early treatment options for COVID-19 in two trial arms. Another study called Repurposing Anti-Infectives for COVID-19 Therapy (ReACT) — coordinated by the non-profit foundation Medicines for Malaria Venture — will test the safety and efficacy of repurposed drugs in South Africa. But regulatory challenges, a lack of infrastructure and difficulties with recruiting trial participants all present major hurdles to such efforts.
“We have a broken health-care system in sub-Saharan Africa,” says Samba Sow, the national principal investigator for ANTICOV in Mali. That makes the trials hard but all the more necessary, particularly for identifying drugs that will help people during the earliest stages of disease and prevent hospitalizations. For him and many others working on the disease, it is a race against death. “We can’t wait until patients become severely ill,” he says.
 The Independent Uganda: You get the Truth we Pay the Price
The Independent Uganda: You get the Truth we Pay the Price



