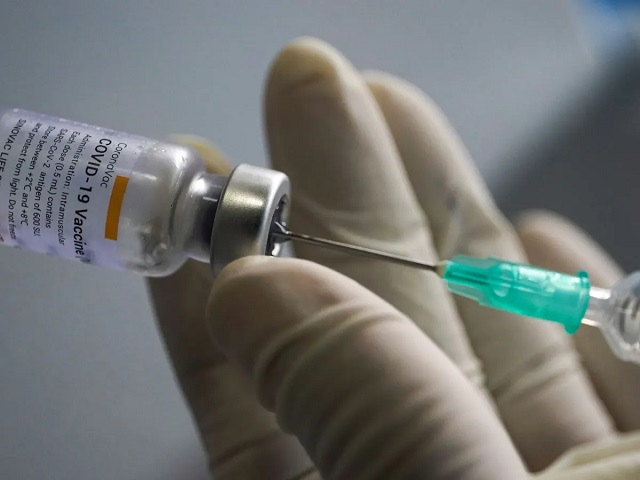
Johannesburg, South Africa | Xinhua | The South African Health Products Regulatory Authority (SAHPRA) on Saturday announced that it had authorized the use of the CoronaVac COVID-19 vaccine developed by Chinese pharmaceutical company Sinovac Biotech.
The latest vaccine approval was based on the “safety, quality and efficacy data” submitted to the regulator between March 22 and June 22, it said in a statement.
“The authorisation is subject to a number of conditions. Specifically, the applicant is required to submit the final results of ongoing clinical studies,” said the authority. “SAHPRA also took account of the World Health Organization Emergency Use Listing report on this vaccine.”
“In addition, the conditions require the submission of periodic safety updates in accordance with SAHPRA guidance, and conformance with pharmacovigilance activities as outlined in the approved risk management plan,” it said.
*****
XINHUA
 The Independent Uganda: You get the Truth we Pay the Price
The Independent Uganda: You get the Truth we Pay the Price


