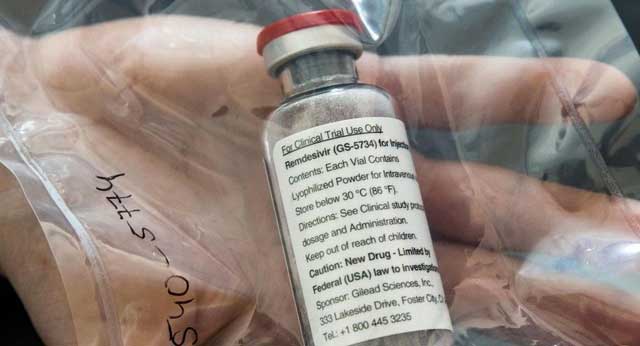
Kampala, Uganda | THE INDEPENDENT | A latest clinical trial in the United States shows that Remdesivir drug is effective in the treatment of the novel coronavirus. The experimental drug by the Gilead Sciences Inc has been under clinical trial treating COVID-19 patients for the last two weeks in the United States.
The US government’s National Institute of Allergy and Infectious Diseases- NIAID on Wednesday in a statement said preliminary data show patients who received Remdesivir recovered faster than similar patients who received placebo.
The National Institute of Allergy and Infectious Diseases trial reportedly found that Remdesivir Accelerates Recovery from Advanced COVID-19 and that patients had a 31 per cent faster time to recovery than those who received placebo.
The trial (known as the Adaptive COVID-19 Treatment Trial, or ACTT) involving 1,063 patients began on February 21. It was sponsored by the National Institute of Allergy and Infectious Diseases (NIAID). It is the first clinical trial launched in the United States to evaluate an experimental treatment for COVID-19.
Remdesivir is widely considered to be a front-runner among the investigational therapies being tested as treatments for COVID-19.
But another study accessing the same drug seemed to dispute finding by the US body in charge of Allergy and Infectious Disease. Findings from a separate study in China, published on Wednesday in the British Medical Journal Lancet, found that the therapy did not lead to clinical improvement in patients in China.
It said Treatment did not speed recovery in that study, which was stopped after only 237 of a planned 453 patients were enrolled.
However, a statement attributed to Anthony Fauci, the director of NIAID by several US-based media quoted him saying that data from the study are a “very important proof of concept” and that there was a reason for optimism.
Fauci who has in the past contradicted President Donald Trump’s claims over COVID-19 treatments and vaccines allegedly cautioned that the data were not a “knockout.” At the same time, the study achieved its primary goal, which was to improve the time to recovery, which was reduced by four days for patients on Remdesivir.
Dr Anthony Fauci said the study showed the drug Remdesivir reduced the time it took coronavirus patients to recover by 31 per cent.
Gilead on Wednesday did release data from its own study of Remdesivir in patients with severe Covid-19. Merdad Parsey, Gilead’s chief medical officer, in a statement said “Unlike traditional drug development, we are attempting to evaluate an investigational agent alongside an evolving global pandemic.
Multiple concurrent studies are helping inform whether Remdesivir is a safe and effective treatment for COVID-19 and how to best utilize the drug,” The drug is given intravenously and is designed to interfere with the virus’s ability to copy its genetic material.
In animal tests against Severe Acute Respiratory Syndrome-SARS and Middle East Respiratory Syndrome (MERS), diseases caused by similar coronaviruses, the drug helped prevent infection and reduced the severity of symptoms when given early enough in the course of illness. But it is not yet approved anywhere in the world for any use.
Remdesivir, developed by Gilead Sciences Inc., is an investigational broad-spectrum antiviral treatment
administered via daily infusion for 10 days. It has shown promise in animal models for treating SARS-CoV-2 (the virus that causes COVID-19) infection and has been examined in various clinical trials.
********
URN
 The Independent Uganda: You get the Truth we Pay the Price
The Independent Uganda: You get the Truth we Pay the Price