Move is part of wide programme that involves routine multivalent meningitis, human rabies, and hepatitis B birth dose vaccination
ANALYSIS | THE INDEPENDENT | In a historic step, preventive Ebola vaccination will become the norm in the highest-risk countries, courtesy of GAVI, the public-private partnership Vaccine Alliance that brings together developing country and donor governments, the World Health Organization, UNICEF, the World Bank, the vaccine industry, technical agencies, civil society, the Bill & Melinda Gates Foundation and other private sector partners.
Gavi will also support lower-income countries for routine administration of human rabies vaccine for post-exposure prophylaxis, as well as multivalent meningococcal conjugate and hepatitis B birth dose vaccines
Gavi announced on June 13 that the lower-income countries it supports can now apply to introduce four additional vaccines: preventive Ebola, human rabies vaccine for post-exposure prophylaxis, multivalent meningococcal conjugate and hepatitis B birth dose.
These four vaccine programmes were previously approved by the Gavi Board but put on hold either due to the COVID-19 pandemic (in the case of human rabies for post-exposure prophylaxis and hepatitis B birth dose); pending availability of suitable products (multivalent meningococcal conjugate); or appropriate policy recommendations (preventive Ebola).
Gavi said the latest expansion of its vaccine portfolio is in line with its commitment to ensure lower-income countries have access to impactful vaccines as soon as possible.
The portfolio will be further expanded in Gavi’s next strategic period, from 2026–2030, which will seek to protect more people, against more diseases, faster than ever before.
Historic progress against Ebola
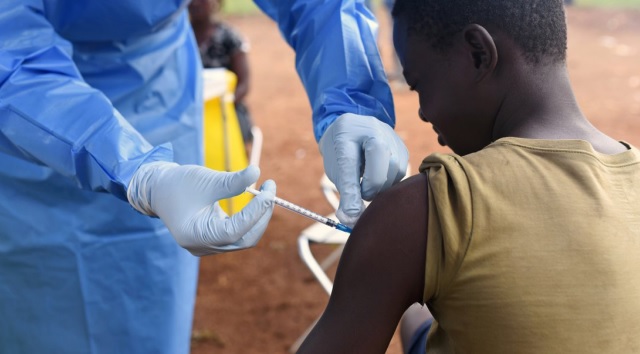
Gavi said its move to begin funding preventive vaccination against Ebola in countries most at risk of outbreaks of the deadly viral disease, Ebola, is an historic milestone for global health security and the protection of health care workers and frontline workers.
The move was made possible by a decision by the World Health Organisation’s Strategic Advisory Group of Experts on Immunization (SAGE) in May to officially recommend the use of the two licensed Ebola vaccines for preventive use in populations at high risk of being exposed to Ebola through their duties as frontline care providers and in response to outbreaks. The decision was based on new data on effectiveness, duration of protection and supply availability.
Gavi first committed to supporting Ebola vaccination in 2014, during the deadly 2014–2016 West Africa outbreak – thereafter facilitating the use of investigational doses for outbreak response and accelerating the pathway to prequalification and the establishment of a global stockpile, which was launched in 2021. In addition to supporting outbreak response from the Gavi-funded global stockpile, Gavi will now make available vaccines for preventive vaccination, and will continue to additionally fund operational costs for vaccination delivery in lower-income countries.
Ebola virus is a rare but severe illness in humans, with the average Ebola case fatality rate around 60% – in some cases much higher depending on the response to an outbreak. However, the availability of safe and effective vaccines has dramatically reduced the number of cases and deaths during outbreaks – helping to bring them quickly under control.
The additional support for preventive vaccination of those at highest risk is significant, as data increasingly indicates that, in addition to risk of spillover events from infected animals to humans, viral resurgence in survivors of Ebola virus disease can also trigger new outbreaks several years later. Preventive vaccination will ensure critical frontline professionals are already protected against infections and death before outbreaks begin – saving lives and avoiding the disruption of services in health care facilities – thus decreasing the risk of further spread among communities.
Ebola response
In 2014, during the deadly 2014–2016 West Africa Ebola outbreak, which took over 11,000 lives and created global health security concerns as cases spread to countries outside the African continent, Gavi announced its commitment to purchase any suitable vaccine candidates. Subsequently, Gavi invested in the acceleration of development of vaccines and the global stockpile – to ensure vaccines were available to countries as quickly as possible.
In 2016 Gavi signed an Advance Purchase Commitment (APC) with Merck that enabled 300,000 investigational doses to be deployed in outbreaks, pending the availability of a WHO-prequalified product. In 2019, upon recommendation by WHO SAGE, Gavi formally approved the launch of an Ebola vaccination programme and establishment of a global Ebola vaccine stockpile of 500,000 doses. The stockpile was established in 2021, once vaccines received WHO prequalification. In approving an Ebola vaccination programme, Gavi also committed to supporting preventive vaccination in the countries at highest risk, pending a better understanding about the durability of the immunological protection of the vaccine and a WHO SAGE recommendation for preventive use – both of which are now in place.
To date, more than 130,000 doses of the Merck ERVEBO® vaccine from the global stockpile have been shipped for preventive vaccination campaigns in the Democratic Republic of the Congo, Guinea-Bissau and Uganda, in addition to the 7,000 vaccines shipped for outbreak response vaccination. The International Coordinating Group (ICG) on Vaccine Provision, hosted by WHO, makes decisions on allocation and deployment of Ebola vaccines from the global stockpile based on country requests for outbreak response; countries can now apply to Gavi for preventive vaccination support.
Gavi’s role is to fund doses, transport and vaccination activities, while UNICEF manages procurement of doses and delivery of vaccines to countries. Gavi also funds global stockpiles of cholera, meningococcal and yellow fever vaccines, and in the future will look to establish stockpiles of vaccines against mpox and hepatitis E.
ICG had approved using these doses preventively based on strong evidence: successful clinical trials, real-world use showing high effectiveness and safety, and existing interim SAGE recommendations to use the vaccine in advance for at-risk populations. Consequently, given availability of doses in the stockpile, and with Gavi and WHO support, some frontline service providers and health workers have already received a single dose. With the new SAGE recommendation, this can now become standard practice.
“Gavi’s ability as an Alliance to protect health and save lives hinges on its ability to ensure vaccines are accessible, as quickly as possible, to who that need them the most. The new programmes launching today demonstrate the impact of this work,” said Dr Sania Nishtar, CEO of Gavi, the Vaccine Alliance. “For example, Ebola is a terrible disease that can lay waste to whole communities. In one decade, we have been able to progress from having no approved vaccines during a deadly outbreak, to having a global stockpile that has helped cut down cases and deaths – and now vaccines even used preventively to protect those at highest risk.”
New tools against deadly infectious diseases
In July 2023, a new multivalent meningococcal conjugate vaccine that protects against the five main serogroups of meningococcal meningitis impacting Africa – meningococcal serogroups A, C, W, Y, and X – received WHO prequalification. It is the only vaccine that protects against serogroup X. The vaccine, MenFive®, is available in the global Gavi-funded stockpile of meningococcal vaccines and has already been used in campaigns aiming to protect over 5 million people in response to meningococcus serogroups C and W outbreaks in Nigeria and Niger. With the opening of today’s application window, countries at high risk can officially roll out this new vaccine through routine programmes and preventive campaigns.
Gavi’s 2018 Vaccine Investment Strategy (VIS) also identified human rabies vaccines for post exposure prophylaxis (PEP) as a highly impactful vaccine to add to the portfolio. With implementation delayed due to the COVID-19 pandemic, Gavi and partners have been working since 2018 to prepare for the launch of the programme, which will support the provision of human rabies for PEP in Gavi-supported countries which are rabies endemic. Rabies is a serious public health problem in more than 150 countries, mainly in Asia and Africa, causing tens of thousands of deaths each year. Children between 5 and 14 years account for almost half of all fatalities. Once someone is symptomatic after exposure to the rabies virus, the disease is 100% fatal – meaning that vaccination in high-risk areas is critical, especially as access to rabies immunoglobulins is unlikely in most countries.
Similarly, Gavi’s 2018 VIS recommended the inclusion of the hepatitis B birth dose vaccine within the Gavi portfolio. Gavi-supported countries already provide routine vaccination against hepatis B through the pentavalent and hexavalent vaccines, which are delivered to children as part of the primary series of vaccinations. However, increasing evidence has shown that a dose given at birth provides critical additional protection. Hepatitis B kills an estimated 884,000 people a year. Newborn babies are most at risk of hepatitis B; and nine out of ten infected infants will develop chronic hepatitis B, while a quarter develop severe liver disease. Vaccination is critical, because while the virus can be transmitted in body fluids, many infants become infected in the womb or during delivery when their mother is carrying the virus. Children can be silent carriers of the infection that doesn’t become symptomatic until their 40s or 50s, when they discover they have liver failure (as the inflammation causes cirrhosis) or liver cancer.
 The Independent Uganda: You get the Truth we Pay the Price
The Independent Uganda: You get the Truth we Pay the Price



