Relative to a decade ago, patients today have a reduced pill burden, decreased side effects, and improved ease of use, which in turn, has reduced barriers to treatment adherence, leading to better health outcomes and improved quality of life.
Once-daily, single-tablet, multi-class treatment regimens have become an integral part of HIV management over the past decade. These fixed-dose combinations are better tolerated and have not only been associated with greater viral suppression, but by combining as many as four different therapies in a single tablet, they have also dramatically reduced pill burden for patients.
Relative to HIV patients taking two or more pills per day, those on once-daily, single-tablet regimens are more likely to achieve the adherence levels needed to avoid the development of drug resistance.
Moreover, adherent patients on singletablet regimens have been found to experience fewer hospitalisations and lower health care costs.
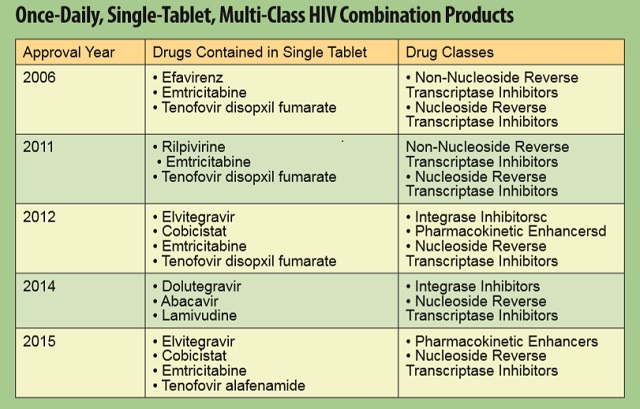
Five such medicines have been approved since 2006 which have simplified dosing, reduced pill burden and maximized potential for successful disease management.
New Classes of HIV Treatments Increase Options for Patients
Three new classes of antiretrovirals have also offered tremendous advancements and expanded treatment options for patients. Because HIV can sometimes become resistant to drugs within the same class—a process known as “cross-resistance”—the availability of additional classes of medicines that target the virus through different mechanisms is important in providing treatment alternatives for patients.
In 2007, raltegravir, the first medicine in a new class called integrase inhibitors, was approved. This class of medicines works by interfering with HIV integrase, an enzyme the virus needs to replicate itself. Because integrase inhibitors do not interfere with human cellular processes, they can also have fewer side effects than other drugs.
Also approved in 2007 was maraviroc, the first of a class of medicines called CCR5 antagonists. The medicine was approved for use in patients infected with a specific type of HIV (the CCR5-tropic virus) and who have evidence of both viral replication and HIV strains resistant to multiple antiretroviral agents.
In 2012, another class of medicines known as pharmacokinetic enhancers joined the arsenal of treatments. These medicines are termed “boosting agents” because they do not have antiviral activity on their own but instead inhibit enzymes in the body that normally metabolise or degrade antiretroviral medicines. Adding these boosting agents to HIV treatment combinations allows antiviral medicines to remain effective in the body for longer periods of time, thereby reducing pill burden and offering simplified dosing schedules. Cobicistat was the first of this class to be approved by the FDA in 2012.
New therapeutic options manage side effects
Over the past decade, advances in HIV treatments have helped to decrease the side effects patients experience. In addition, treatments have become available to help better manage common side effects of antiretroviral treatments. Advances such as these are particularly important for HIV patients as they continue to live longer lives.
Long-term prevention efforts today continue to focus on the development of a preventive vaccine, which many experts believe is the best strategy for controlling or even ending the HIV epidemic. Currently, 33 vaccine candidates are in clinical development, offering tremendous hope for a future without HIV/AIDS.
Then
- Four antiretroviral drug classes were available to patients suppressing the virus through different mechanisms of action.
- Patients required twice-daily dosing of multiple medicines and pill burden remained a challenge.• Patients developing resistance to medicines from existing drug classes were limited in alternative treatment options.
- Patients were limited in treatment options for the management of side effects of antiretroviral therapy.
Now
- Three new classes of antiretroviral medicines are available to patients offering important new options for those failing or developing viral resistance to existing therapies.
- Five once-daily, single-tablet, multiclass combination products significantly reduce pill burden, provide greater efficacy and fewer side effects for patients, thus contributing to improved adherence and successful disease management.
- New treatments are available to help patients manage common and debilitating side effects of antiretroviral treatment.
- Average life expectancy for patients has increased 10 years relative to that seen just a decade ago.
- Prophylactic medications offer to reduce the risk of infection by 92% among high risk individuals and 33 vaccines are in development offering great hope for a future without HIV. HIV/AIDS
QUICK FACTS
- The human immunodeficiency virus (HIV) gradually destroys the body’s immune system by entering and taking over infection-fighting T-cells.
- If left untreated, HIV will attack the immune system until it progresses to acquired immunodeficiency syndrome (AIDS).
- 1.3 million people are currently living with HIV in Uganda, and 35 million globally.
- Antiretroviral drugs for the treatment of HIV suppress the virus— sometimes to undetectable levels— by interfering with viral replication.
- Uganda has 50000 new infections each year Uganda has 26,000 AIDS-related deaths each year
- 73% of adults who need it are on ART
- 68% of children who need it are on ART
****
 The Independent Uganda: You get the Truth we Pay the Price
The Independent Uganda: You get the Truth we Pay the Price




Can you name any newly approved ART medication in past 10 years is SUPERIOR than classical three drugs regimen ( Lamivudin+Efavirenz+tenofovir disoproxil fumarate)?
How can be judged the progress made by researchers in past 10 years?
sand to my email is a request thanks
Where exactly aids originated?if the answer is available,then cure is there!!!!?or probably somebody must be held responsible.