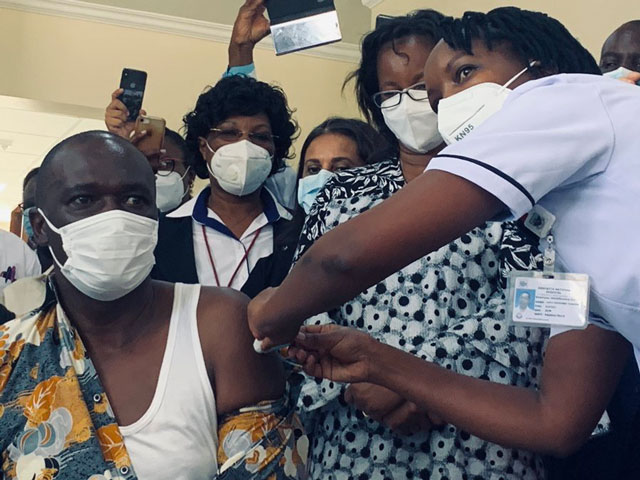
Geneva, Switzerland | THE INDEPEDNENT | The World Health Organization, the vaccines alliance GAVI and Coalition for Epidemic Preparedness Innovations CEPI have launched one of the most ambitious inoculation delivery plan in history, dubbed COVAX.
The plan is to ensure the poorer countries in the world are all able to receive vaccines, some of it free, and more at an affordable cost. Over 92 countries in the developing world, mainly in Africa will benefit.
At the Global Vaccine Summit last year, Gavi launched the COVID-19 Vaccines Advance Market Commitment (COVAX AMC) as the first building block of the COVAX Facility. The Gavi COVAX AMC is the innovative financing instrument that will support the participation of 92 low- and middle-income economies in the COVAX Facility – enabling access to donor-funded doses of safe and effective COVID-19 vaccines. The AMC, combined with additional support for country readiness and delivery, will make sure the most vulnerable in all countries can be protected in the short term, regardless of income level.
COVAX has even addressed how the COVAX Facility will address potential liability,
and compensation in the event of unexpected serious adverse events (SAE), arising from the manufacture, storage, transportation and administration of COVID-19 vaccines for AMC Countries.
******
READ THE PLAN BELOW
Following accelerated development, the delivery of COVID-19 vaccines at virtually the same time in hundreds of countries and territories will be the fastest and the largest deployment of a novel vaccine in history.
All vaccines made available or procured through the COVAX Facility will have received regulatory approval or an emergency use authorization, allowing their general availability (the “Vaccines”). However, even under normal circumstances, vaccines that are approved for general use may nevertheless, in rare cases, cause unexpected serious adverse events (SAEs). Those involved in their manufacture, distribution and administration can normally get insurance to cover this risk.
Given the unprecedented nature and scale of the COVID-19 pandemic, however, normal insurance will not be available from the outset. The lack of such coverage may limit or delay global access to lifesaving Vaccines as manufacturers are reluctant to deliver COVID-19 Vaccines if this risk is not addressed. They are also, in the first instance, looking to countries receiving and deploying the Vaccines – including via bilateral deals – to indemnify them against product liability claims (as was the case at the time of the H1N1 pandemic). Equally, people receiving Vaccines who suffer unexpected SAEs associated with a Vaccine or its administration deserve compensation.
The COVAX Facility is doing everything possible to find a practical solution to make sure this indemnification requirement is not a barrier to access, particularly to low and middle-income countries. This solution has to be one that can be implemented within the short time frame available before deployment of Vaccines, is equitable to all stakeholders and mitigates the anticipated financial risks to AMC countries. The COVAX Facility is developing a system to provide compensation to those individuals in any of the 92 economies to be supported by Gavi, the Vaccine Alliance (Gavi) under the COVAX Advance Market Commitment (the “AMC Group”) that suffer unexpected SAEs associated with such Vaccines or their administration. In addition, recognizing that countries and territories will be required to indemnify the manufacturer, the COVAX Facility is exploring backstopping guarantees for these indemnification obligations for the 92 participants in the AMC Group .
It is critical to note that COVAX will not compromise on safety and efficacy of COVID-19 Vaccines supplied through the COVAX Facility, and will, in addition to all the rigorous processes that will be followed by COVAX, rely on regulatory authorities to ensure that is the case.
Against the background provided above, this document provides an overview of how the COVAX Facility will address potential liability and compensation to Vaccine recipients in the event they suffer unexpected serious adverse events, arising from the manufacture, storage, transportation and administration of Vaccines for countries in the AMC Group.
READ FULL DOCUMENT PROPOSAL HERE>>>> BRIEFING-NOTE-Indemnification-and-Compensation-COVAX-AMC-Countries
 The Independent Uganda: You get the Truth we Pay the Price
The Independent Uganda: You get the Truth we Pay the Price