New Delhi, India | XINHUA | Human trials for developing an indigenous vaccine against COVID-19 are going on in full swing in India.
Two companies — Bharat Biotech and Zydus Cadila — are currently carrying out human trials in six cities across the country.
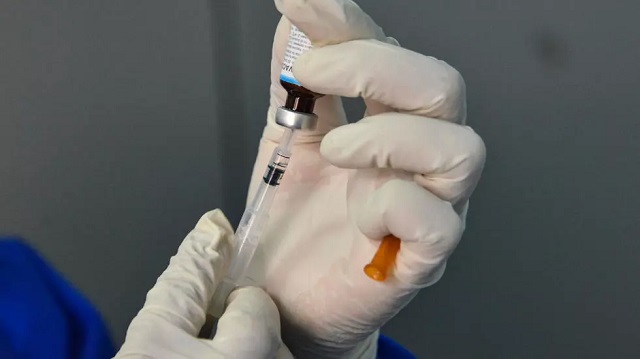
On Friday a 30-year-old man was administered the first dose of the under-trial COVID-19 vaccine namely Covaxin Bharat Biotech at the prestigious All India Institute Of Medical Sciences (AIIMS). The man was administered 0.5 ml intramuscular injection during the part of the primary trial.
“The first dose of Covaxin was administered to a 30-year-old man, the first volunteer, who is a resident of Delhi. He was screened two days ago and all his health vital parameters were normal. He does not have any co-morbid conditions or any pre-existing illness,” Dr Sanjay Rai, who works within the Community Medicine Department, said.
“After the vaccination, the man was in close observation for two hours. There was no sudden side-effect observed.”
Rai, who is also the principal investigator of the research, said the subject will be closely monitored for a week.
“The volunteer has been allowed to go home for now and he will be examined again after two days,” he said.
According to officials, 500 volunteers have already registered for the trial for developing the COVID-19 vaccine at the AIIMS.
More volunteers will be vaccinated on Saturday.
Bharat Biotech and Zydus were granted permission for Phase I and II clinical trials and administered the first doses of their vaccines to volunteers on July 15.
A third vaccine candidate, developed by Oxford University, is soon to be tested in India. Serum Institute, which is in a manufacturing partnership with the UK’s Astra Zeneca, has said it will begin human trials as soon as it receives regulatory approval. This vaccine is currently at the third level of human clinical trial stage.
Bharat Biotech’s Covaxin, developed in collaboration with India’s top health research body — the Indian Council of Medical Research (ICMR) and the National Institute of Virology (NIV), will be tested at 12 hospitals ( including AIIMS, Delhi, Patna and Post-Graduate Institute of Medical Sciences -PGIMS, Rohtak) in 12 different cities.
AIIMS Patna, which started human trials of Covaxin on 11 volunteers on July 15, has not encountered any major side-effects in volunteers. The results of the first dose are yet to come in.
Likewise, the Post-Graduate Institute of Medical Sciences (PGIMS) in Rohtak has readied 20 volunteers and administered the first dose to three people on July 17 and none of them have showed any adverse impact of the vaccine. The second phase of human trials will begin soon.
At SRM Medical College Hospital and Research Centre in Kancheepuram, near Chennai, human trials began on Thursday with two volunteers being administered 0.05 ml of the vaccine each. They will get the next dose on August 5.
The first phase will involve over 500 volunteers, all healthy and between the age of 18 and 55 years with no co-morbidities.
Covaxin trials have already begun in Hyderabad, Patna, Kancheepuram, Rohtak, and now Delhi, to be followed by Nagpur, Bhubaneshwar, Belgaum, Gorakhpur, Kanpur, Goa and Visakhapatnam.
Testing of Zydus’s candidate, ZyCoV-D, is currently limited to its research centre in Ahmedabad and will be subsequently extended to multiple cities. Indian government’s department of biotechnology has partially funded the development of Zydus’s vaccine ZyCov-D.
The human clinical trials of the indigenous COVID-19 vaccines are being carried out at various levels in the country to identify the efficacy of these potential vaccines.
Officials said the government has assured to provide all the necessary support for the development of vaccine.
On Friday, India’s multinational pharmaceutical company Cipla announced it has received regulatory approval from the drug controller general of India (DCGI) for launching Favipiravir in the country to be sold under the brand name Ciplenza for restricted emergency use for the treatment of coronavirus patients. The company said it will commercially launch Ciplenza in the first week of August and each tablet has been priced less than a U.S. dollar.
Ciplenza has been jointly developed by Cipla and CSIR-Indian Institute of Chemical Technology (IICT).
India Saturday said the number of COVID-19 cases in the country have reached 1,336,861 including 31,358 deaths.
Globally India is the third worst-hit country due to COVID-19 pandemic.
******
XINHUA
 The Independent Uganda: You get the Truth we Pay the Price
The Independent Uganda: You get the Truth we Pay the Price



With God almighty everything is possible and we keep praying for more of God’s grace.