Brussels, Belgium | Xinhua | The European Medicines Agency (EMA) said on Thursday that there was “insufficient evidence” for it to recommend the administration of a second booster vaccine against COVID-19. “At the moment, there is insufficient evidence from clinical trials or real-world evidence that could support our recommendation for the …
Read More »European Commission authorizes fifth vaccine against COVID-19
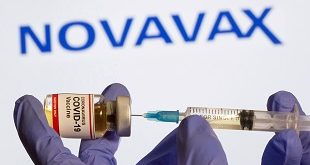
Brussels, Belgium | Xinhua | The European Commission on Monday granted conditional marketing authorization for Nuvaxovid, the fifth COVID-19 vaccine authorized in the European Union (EU). The authorization of the vaccine, developed by Novavax, followed a scientific recommendation based on an assessment of the safety, effectiveness and quality of the vaccine …
Read More »EMA recommends emergency use of Pfizer COVID-19 pill
The Hague, Netherlands | Xinhua | The European Medicines Agency (EMA) on Thursday issued advice on the emergency use of Pfizer’s COVID-19 pill called Paxlovid. The European Union (EU) drug regulator said the medicine, which is not yet authorized in the EU, can be used to treat adults with COVID-19 who …
Read More »EU drug agency recommends 2 anti-COVID-19 medicines
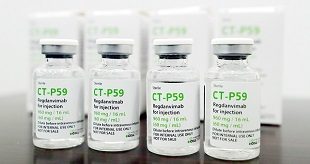
The Hague, Netherlands | Xinhua | The European Medicines Agency (EMA) has recommended the authorization of two medicines for the treatment of COVID-19. The two medicines are Ronapreve made by the Switzerland-based pharmaceutical company Roche, and Regkirona made by South Korean drug company Celltrion, the agency said in a statement on …
Read More »BioNTech/Pfizer seek approval for COVID-19 vaccine use in adolescents
Berlin, Germany | Xinhua | German biotechnology company BioNTech and U.S. company Pfizer have submitted a request to the European Medicines Agency (EMA) for the approval of an extension of their COVID-19 vaccine use in adolescents aged 12 to 15, the two companies announced on Friday. The submission was based on …
Read More »Coronavirus vaccine may be ready in a year: EU agency
The Hague, Netherlands | AFP | A vaccine for the novel coronavirus could be ready in a year’s time under an “optimistic” scenario, based on trials that are underway, the European Medicines Agency said Thursday. The Amsterdam-based EU agency also played down fears expressed by the WHO that the virus might …
Read More »‘Serious side effects’ from Trump-backed virus drugs: EU agency
The Hague, Netherlands | AFP | The European Medicines Agency on Thursday added its voice to growing concern about an anti-malarial drug widely touted as a potential cure for the COVID-19 disease, warning about fatal side effects. Beneficial effects of chloroquine and hydroxychloroquine, which count US President Donald Trump as a major backer, have “not …
Read More » The Independent Uganda: You get the Truth we Pay the Price
The Independent Uganda: You get the Truth we Pay the Price