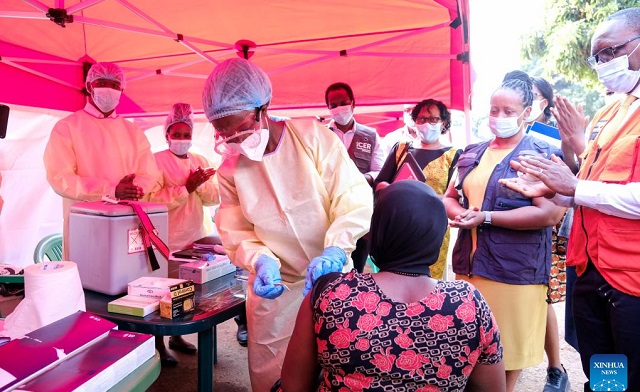
Kampala, Uganda | THE INDEPENDENT | The Ministry of Health has started rolling out the Ebola Sudan Trial Vaccine to high-risk populations following the recent outbreak in Uganda. This comes after the country registered its eighth outbreak of the Ebola Sudan strain, in which a health worker from Mulago Hospital succumbed to the disease.
Professor Bruce Kirenga, the trial’s principal investigator, stated that there are 2,460 doses of the vaccine available, which are being administered under a clinical trial. Participants, who are contacts of confirmed cases, must provide informed consent after fully understanding the information about the vaccine before voluntarily participating.
Kirenga added that the available vaccine is still under trial and does not yet have an official name but is code-named RVSV or V921. It is specifically designed to target the current strain of Ebola Sudan. There are several strains of the Ebola virus, including Ebola Zaire, which already has a licensed vaccine.However, the Ebola Sudan strain, responsible for the current outbreak, does not yet have a licensed vaccine for outbreak response. Additionally, Kirenga noted that Uganda has the J&J vaccine, which offers protection against Ebola Zaire and a partial response to Ebola Sudan. However, its formulation is not suitable for outbreak response but rather for preventive measures.
Following the 2022 Ebola outbreak, the Ministry of Health rolled out the J&J vaccine to frontline healthcare workers. Kirenga explained that while the J&J vaccine takes two and a half months to provide protection, the Ebola Sudan trial vaccine works much faster, offering protection within seven to nine days. This makes it a valuable tool for safeguarding high-risk individuals during an outbreak.
Dr. Daniel Kyabayinze, the Director of Public Health at the Ministry of Health, emphasized that Uganda is using this opportunity to gather sufficient evidence to determine the vaccine’s effectiveness for future use. Dr. Kyabayinze expressed gratitude to the volunteers, stressing that the vaccine is not intended for the general public but only for selected contacts who have been exposed to Ebola.
Dr. Mike Ryan, the Deputy Director-General of the WHO, praised Uganda and its partners for their efforts in the first deployment of the Ebola Sudan trial vaccine for evaluation. Ryan assured Ugandans that the trial vaccine has been developed following the highest scientific standards. He acknowledged the Ugandan government, Makerere University, and the Uganda Virus Research Institute (UVRI) for their proactive planning, which ensured readiness in the event of an Ebola Sudan outbreak—an effort that has now been demonstrated.
Dr. Kasonde Mwinga, the World Health Organization (WHO) Representative for Uganda, commended the Ugandan government for launching the Ebola vaccine trial. She added that this vaccine is just one of the tools being used to combat the outbreak, but WHO is employing various strategies in collaboration with the Ministry of Health.
Kasonde also called on development partners to support Uganda’s efforts in the fight against Ebola Sudan. There are three main types of Ebola: Ebola Sudan, which is responsible for the current outbreak in Uganda; Ebola Zaire, which already has a licensed vaccine; and Ebola Bundibugyo, which was first identified in Uganda. Uganda has experienced eight Ebola outbreaks, all of which have been successfully managed. However, this is the first time an Ebola outbreak has started in the capital, Kampala.
****
URN
 The Independent Uganda: You get the Truth we Pay the Price
The Independent Uganda: You get the Truth we Pay the Price



