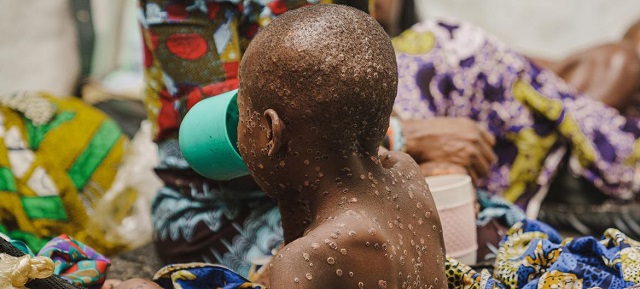
The decision is a crucial step in getting shots to the Democratic Republic of Congo, the center of the outbreak
ANALYSIS | STEPHANIE NOLEN | The World Health Organization has given its authorization to a first vaccine to protect against mpox, a decision announced in such haste on Friday that it caught even the head of the company that makes the vaccine by surprise.
The vaccine, made by the Danish company Bavarian Nordic, has been approved by the regulatory authorities in Europe as well as the United States and other high-income countries since a global mpox outbreak in 2022. But low- and middle-income countries rely on the W.H.O., through a process called prequalification, to determine which drugs, vaccines and health technologies are safe and efficient uses of limited health funding, and the organization had declined to act until now.
The W.H.O. had come under increasing criticism for declaring a global public health emergency for mpox last month without giving a vaccine that prequalification stamp of approval, or a more provisional form of approval called emergency use authorization. Bavarian Nordic first submitted its safety and effectiveness data on the vaccine, called Jynneos, to the W.H.O. in 2023.
The W.H.O. had defended its slow pace of review, saying that it needed to subject the vaccine to careful study because it, and two others that have been used to protect against mpox, were originally designed as smallpox immunizations, and because delivering it in low-resource settings such as Central Africa would involve factors different from those relating to its use in high-income countries.
But on Friday morning, the W.H.O. suddenly said it was authorizing the shot.
“This first prequalification of a vaccine against mpox is an important step in our fight against the disease, both in the context of the current outbreaks in Africa, and in future,” Dr. Tedros Adhanom Ghebreyesus, the W.H.O. director general, said in a statement.
Paul Chaplin, Bavarian Nordic’s chief executive, said he was among the many who had been caught off guard.
“We’ve got there eventually — I don’t know quite how,” he said. “But it’s good news. It’s going to make the regulatory pathway much easier.”
Mpox was first identified more than 50 years ago in the Democratic Republic of Congo and has been endemic there ever since. While the global spread that started in 2022 waned in 2023, people in Congo have continued to become infected. A new version of the virus, one that is sexually transmissible, was identified there in 2023, and there have been more than 21,000 suspected mpox cases, and 700 deaths, this year.
No vaccines administered in DRC
However, no vaccines have yet been publicly administered in Congo.
About 245,000 donated shots, from the European Union, the United States and Bavarian Nordic, began to arrive in the capital, Kinshasa, last week. The Congolese government has said it hoped to begin to distribute them by Oct. 2.
The Jynneos prequalification decision vaulted over a planned meeting next week of a W.H.O. committee that is set to evaluate the vaccine for emergency use.
Prashant Yadav, an expert on health technology supply chains and a professor of technology and operations management at the French business school INSEAD, said the W.H.O. decision to authorize the vaccine now was both surprising and admirable.
“It’s not routine for them to do such an expedited approval, and I commend them for doing this,” he said.
The Africa Centres for Disease Control and Prevention declared its own public health emergency for mpox, before the W.H.O. did so, and African health ministers have expressed frustration at the pace of response from the global organization.

In its announcement on Friday, the W.H.O. said that it was authorizing the vaccine for adults but that it could be used at the discretion of health care providers for children and adolescents under age 18.
More than half of the mpox cases in Congo this year have been in children, according to UNICEF. The W.H.O.’s expert committee on vaccination said in August that the benefits of using the vaccine for children at high risk of exposure outweighed the risk.
In late August, the W.H.O. instructed Gavi, an international organization that funds vaccines for low-income countries, and UNICEF, which procures the shots, to issue a tender for mpox vaccines even before the emergency use license had been considered by its experts.
Bavarian Nordic submitted a bid, Mr. Chaplin said, and could supply two million doses of the vaccine this year and an additional 11 million in 2025. The Africa C.D.C. says that as many as 10 million doses of the vaccine could be required to respond to an outbreak that now involves more than a dozen African countries, including Burundi and Uganda, which have never before reported mpox.
Vaccine given in two shots
The vaccine must be given in two shots for strongest protection, although the W.H.O. said use of a single shot should be considered in emergency situations where supply is constrained. Bavarian Nordic charged about $115 per shot to higher-income countries in 2022; Mr. Chaplin, the chief executive, said the price charged to Gavi would depend on the volumes ordered.
Gavi has a dedicated “first response fund” of money meant for purchasing vaccines in emergencies such as this one and will face pressure to make an order large enough that African countries will have sufficient supply of the vaccine.
Getting supplies to the countries is only one challenge. These vaccines require complex logistics of transport, temperature control, staff training and community education, and the production of the shots is only the first piece of trying to protect against the virus.
“While we remain in a supply-constrained environment, an important focus now is to translate deliveries of vaccines into countries into vaccinations,” said Derrick Sim, Gavi’s director of vaccine markets and health security. “One of the lessons from the Covid-19 pandemic is that vaccine supply needs to be matched with the timing of countries’ vaccination programs.”
Indemnity will be a separate challenge. Bavarian Nordic has liability insurance for the use of the vaccine in adults, but that insurance will not cover off-label pediatric use.
“Use in children would need to be included in the emergency use authorization for the individual countries,” Mr. Chaplin said. “Or the organization using the product off label would have to take the responsibility.
****
Source: New York Times
 The Independent Uganda: You get the Truth we Pay the Price
The Independent Uganda: You get the Truth we Pay the Price